 Children born with cardiac and other congenital birth defects have potentially been linked to use of the SSRI class of antidepressant drugs, including Lexapro (Generic: escitalopram). Research suggests serious Lexapro side effects, including the increased risk of congenital heart defects and other congenital birth defects. This link between SSRI antidepressants like Lexapro (escitalopram) and congenital heart defects has many professional concerned that women should not be prescribed the medication Lexapro (escitalopram) or other SSRIs during pregnancy. In addition to serious congenital Heart Birth Defects, SSRIs like Lexapro, Celexa, Prozac, Zoloft and Paxil have also been linked to increased risk of Persistent Pulmonary Hypertension of the Newborn (PPHN).
Children born with cardiac and other congenital birth defects have potentially been linked to use of the SSRI class of antidepressant drugs, including Lexapro (Generic: escitalopram). Research suggests serious Lexapro side effects, including the increased risk of congenital heart defects and other congenital birth defects. This link between SSRI antidepressants like Lexapro (escitalopram) and congenital heart defects has many professional concerned that women should not be prescribed the medication Lexapro (escitalopram) or other SSRIs during pregnancy. In addition to serious congenital Heart Birth Defects, SSRIs like Lexapro, Celexa, Prozac, Zoloft and Paxil have also been linked to increased risk of Persistent Pulmonary Hypertension of the Newborn (PPHN).
Antidepressant Drugs Include:
What is Lexapro?
Lexapro (Generic: escitalopram) is an antidepressant drug of the selective serotonin reuptake inhibitor (SSRI) class. It has FDA approval to treat major depression, and is prescribed off-label for a number of anxiety conditions. Citalopram, (Brand name: Celexa), originally created in 1989 by the pharmaceutical company Lundbeck was the forerunner of Lexapro. The patent for Celexa expired in 2003, allowing other companies to legally produce generic versions. Lundbeck then released an updated formulation called escitalopram (Brand name: Lexapro), which is the S-enantiomer of the racemic citalopram, and acquired a new patent for it. In the United States, Forest Labs currently manufactures and markets the drug.
Lexapro (escitalopram) is approved to treat the symptoms of major depression and generalized anxiety disorder (GAD; excessive worry and tension that disrupts daily life and lasts for 6 months or longer). It works by increasing the amount of serotonin, a natural substance in the brain that helps maintain mental balance. Lexapro (escitalopram) and other SSRIs like Prozac, Paxil and Zoloft can also be used to treat hot flashes. Escitalopram is sold under the brand-name Lexapro (U.S. and Canada, Forest Laboratories, Inc.). Lexapro (escitalopram) is available in 5 mg, 10 mg and 20 mg tablets, as well as an oral solution.
Lexapro Birth Defects
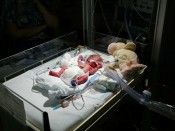 Lexapro (escitalopram) and other SSRI antidepressants has been linked to increased risk of serious birth defects including Primary Pulmonary Hypertension of the Newborn (PPHN) and congenital heart defects. A study published in the New England Journal of Medicine revealed that newborns whose mothers took SSRI antidepressants such as Lexapro during pregnancy were six times more likely to be born with the cario-pulmonary birth defect PPHN than those whose mothers did not take prescribed antidepressants – PPHN rose as high as 12 cases per 1,000 births.
Lexapro (escitalopram) and other SSRI antidepressants has been linked to increased risk of serious birth defects including Primary Pulmonary Hypertension of the Newborn (PPHN) and congenital heart defects. A study published in the New England Journal of Medicine revealed that newborns whose mothers took SSRI antidepressants such as Lexapro during pregnancy were six times more likely to be born with the cario-pulmonary birth defect PPHN than those whose mothers did not take prescribed antidepressants – PPHN rose as high as 12 cases per 1,000 births.
Another study published in Pediatrics (February, 2004) found that pregnant women who used SSRIs had an increased risk of giving birth to babies with abnormal heart rhythms, unusual sleeping patterns, disrupted neurological development and problems with alertness.
A study summarized on Web MD reported that newborns exposed to SSRI antidepressants such as Lexapro by their pregnant mothers late in pregnancy were twice as likely to risk admission to special-care nurseries and twice as likely to suffer respiratory complications serious enough to require hospital ventilation procedures.
In addition to PPHN, SSRI antidepressants like Lexapro, Celexa, Paxil, Prozac and Zoloft have been linked to severe congenital heart defects, including: atrial septal defects (ASD – hole in the heart), ventricular septal defects (VSD – hole in the heart), tetrology of fallot (ToF), hypoplastic left heart syndrome (HLHS), transposition of the great arteries (TGA or TOGA), patent ductus arteriosus (PDA), total anomalous pulmonary venous return (TAPVR), coarctation of the aorta (CoA), double outlet right ventricle (DORV), and Shone’s Complex. These injuries range from minor heart murmurs not requiring surgery, all the way to the most severe congenital heart defects, requiring multiple surgeries or potentially a complete heart transplant in order to save the life of the child.
Children born with cardiac and other congenital birth defects have potentially been linked to use of the SSRI class of antidepressant drugs, including Lexapro (Generic: escitalopram). Research suggests serious Lexapro side effects, including the increased risk of congenital heart defects and other congenital birth defects. This link between SSRI antidepressants like Lexapro (escitalopram) and congenital heart defects has many professional concerned that women should not be prescribed the medication Lexapro (escitalopram) or other SSRIs during pregnancy. In addition to serious congenital Heart Birth Defects, SSRIs like Lexapro, Celexa, Prozac, Zoloft and Paxil have also been linked to increased risk of Persistent Pulmonary Hypertension of the Newborn (PPHN).
Speak to a Lexapro Birth Defect Lawyer
If you took Lexapro during pregnancy and your child was born with a severe birth defect, we encourage you to contact a Lexapro litigation attorney at our law firm immediately. It may be too late to recover from the debilitating effects of Lexapro, but an experienced products liability attorney at the Willis Law Firm can assist you in a legal action against the makers of Lexapro. You are not alone. Join other birth defect victims and their families in speaking up and fighting for your legal rights.
Lexapro Birth Defect Lawsuit
Please fill out our free online legal evaluation form and we will contact you within 24 hours. Please keep in mind that certain states have statutes of limitation that limit the amount of time you have to file a lawsuit or seek legal action. Contact our law firm immediately so that we may explain the rights and options available to you and your family.

